RVCL Clinical Trial Underway
The Clayco Foundation and RVCL.org are actively involved with novel research projects and clinical trials that may benefit patients afflicted with RVCL disorder. Currently, the Foundation is working with several centers of academic excellence, such as Washington University School of Medicine and the University of Pennsylvania, and hopes to continue expanding this network. The current clinical trial supported is a phase 2, multi-site trial testing the drug Crizanlizumab against RVCL disease progression (NCT04611880).
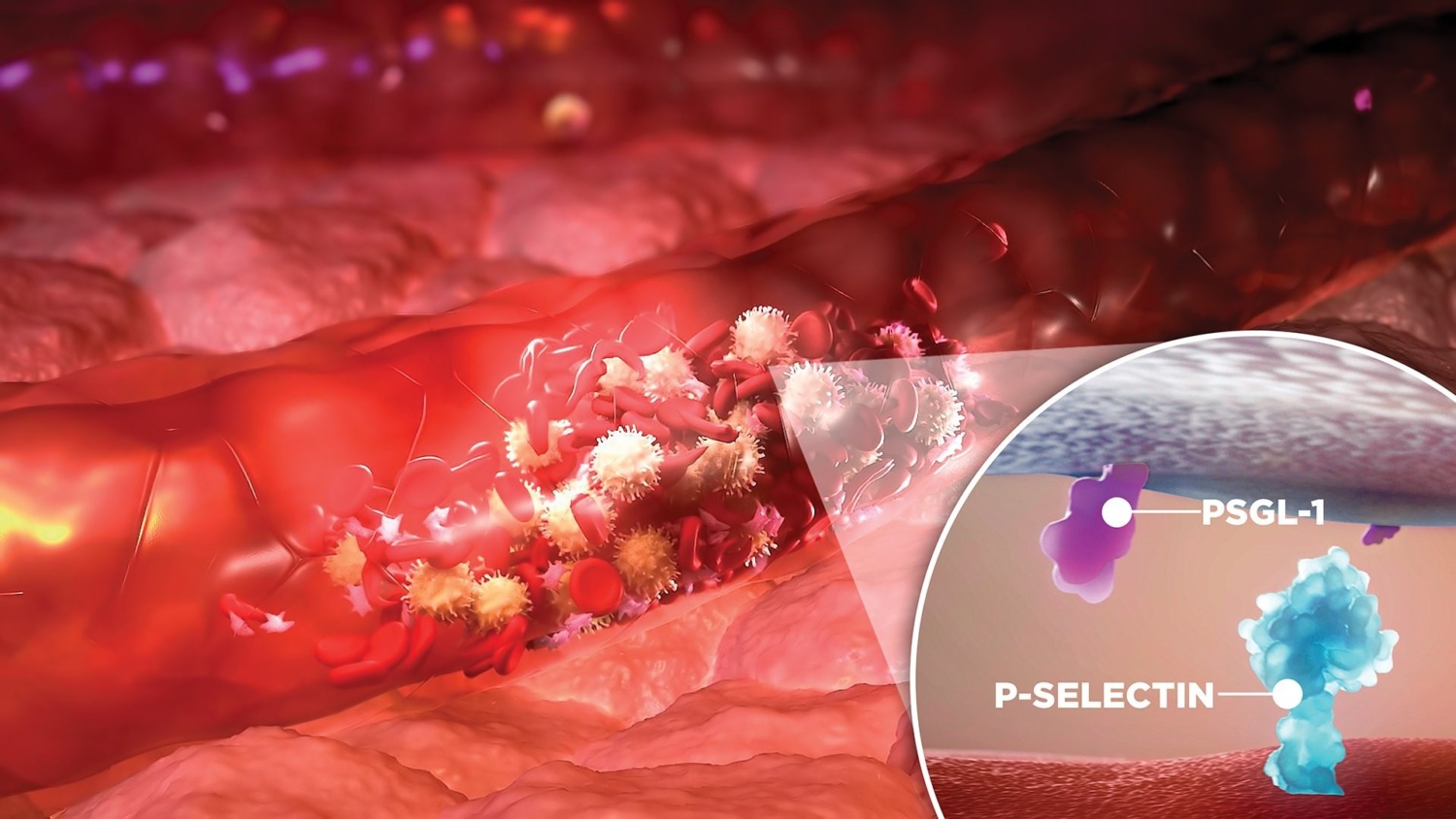
Blood vessels can express a “sticky” protein (called P-Selectin) when needing to recruit immune cells to fight infection. This leads to cellular clusters and brings inflammation to this location. However, in some disease states (such as Sickle Cell Disease), P-Selectin is:
- expressed too much,
- clustering is too thick,
- inflammation is too dysregulated,
- and there is severe damage.
Crizanlizumab (a.k.a. ADAKVEO), a P-selectin inhibitor, (a.k.a. a drug that blocks and inactivates P-Selectin), showed encouraging success in preventing clustering and damage in Sickle Cell Disease patients in clinical studies and was FDA approved in 2019.
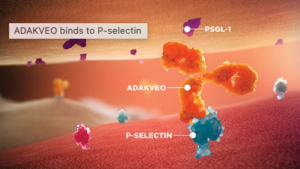
As with Sickle Cell Disease, RVCL shares this clustering abnormality, and therefore it may be possible that Crizanlizumab could also help RVCL patients and delay disease progression. This trial aims to identify if this drug may indeed be a candidate to treat RVCL, and the Foundation is excited to be able to support such a worthy and exciting opportunity.
These images and more information can be found here: Novartis
