Can Correcting the TREX1 Mutation Reverse RVCL?
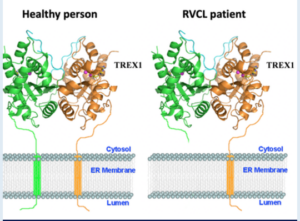
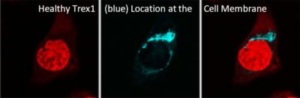
Genetic mutation leading to disease is not a new discovery or phenomenon, and many have heard of such afflictions as Down Syndrome, Cystic Fibrosis, and Huntington’s Disease to name a few. And though RVCL is far rarer, it too is a disease caused by aberrant mutations in the patient’s genetic signature. Specifically, it is typically caused by a random mutation in the transmembrane “anchor” domain of the TREX1 gene which leads to its ‘free-floating’ in the cell (rather than tight regulation at the cell membrane). If this genetic code could be restored, might it be possible to reverse and correct the RVCL disease?
In 1987, the first hints of the Clustered regularly interspaced short palindromic repeat (CRISPR)-Cas system was beginning to be identified. These CRISPR sequences are widespread in archaea and bacteria, and were first discovered in the bacteria E. Coli during genetic studies. It was then noticed that they seem to always be coordinated with the Cas proteins, proteins that can cleave and remove specific (mutated) DNA sequences. While the studies since the foundational discoveries have been impressive and deep, the punchline today is that the system (of an RNA guide to a DNA sequence of interest + an attached protein capable of cutting it out to allow for correct DNA repair) appears useful in treating genetic disorders. Recent publications have highlighted its usefulness in different diseases, including Transthyretin amyloidosis1, Sickle Cell2, and soon to be more3,4. Thus, the question must be asked: Could this CRISPR-Cas system be utilized to repair mutant TREX1 and restore RVCL patients to a healthy stature?
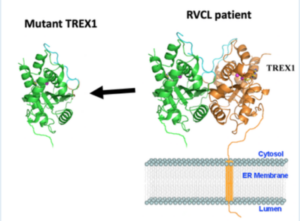
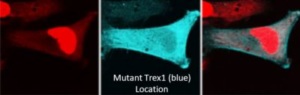
Recent in vitro (a.k.a. in “Petri dishes”) experiments with TREX1 mutant cells and the CRISPR-Cas system has revealed a greater than 90% “gene correction rate” with a less than 10% off-target effect. And though this is only in the lab today and requires much more effort to reach a point of legitimate therapeutic potential, this scientific validation is crucial. Might RVCL be treatable and even possibly curable at its foundational misstep in the TREX1 gene? Time and research will tell…stay tuned!

- Gillmore, J, et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. NEJM 385:493-502. 2021.
- Frangoul H, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and B-Thalassemia. NEJM 384:252-260. 2021.
- Lu L, et al. Application of CRISPR/Cas9 in Alzheimer’s Disease. Frontiers in Neuroscience. 2021.
- Abdelnour, S et al. The Potential of CRISPR/Cas9 Gene Editing as a Treatment Strategy for Inherited Diseases. Frontiers in Cellular Development and Biology.
